Our Journey of Innovation
Our Team
Our team has a passion for advancing the treatment of bleeding disorders and hemorrhage, as well as transforming clinical care.
Our collective experience* has resulted in the discovery, development and commercialization of more than 50 drugs, many of which have held the position of category leader.

Andrea Ashford-Hicks
Chief Executive Officer*
Read more
Ms Ashford-Hicks has more than 20 years of biopharmaceutical industry experience spanning multiple product launches, IND, BLA and NDA approvals as well as business development transactions. Prior to joining Cayuga, she led the BLA approval and commercial launch of RETHYMIC® at Enzyvant Therapeutics. Previously, Ms Ashford-Hicks held roles of increasing responsibility at Johnson & Johnson, Pfizer, Agenus, EMD Serono and Schering-Plough (now Merck). She has degrees in Biology and Finance from the University of Pennsylvania’s College of Arts and Sciences and The Wharton School of Business.

Damien Kudela, PhD
Founder, Chief Science Officer*
Read more
Dr Kudela is the founder of Cayuga and inventor of CAY001. He received his PhD in Materials Chemistry from the University of California, Santa Barbara. He graduated from Cornell University with a BA in Chemistry, cum laude, and a BA in History. He graduated from Deerfield Academy. Prior to founding Cayuga Biotech, Dr. Kudela interned with the US Department of State.

Samina Bari
Corporate and Business Affairs
Read more
Ms Bari has more than 30 years of corporate strategy and operations experience in biopharmaceuticals. Her expertise is in investor strategy, corporate positioning, crisis management and government / public affairs. Previous transactional experience includes the acquisitions of Aimmune by Nestle, Medivation by Pfizer, Pharmacyclics by Abbvie and Ikaria by Mallinckrodt worth more than $40B. Previously, Ms Bari held roles of increasing responsibility at J&J and Pfizer.

Herb Brinkman, PhD
CMC
Read more
Dr Brinkman has over 30 years of experience in the pharmaceutical industry. He has prepared over 25 Chemistry Manufacturing and Control (CMC) sections for multiple IND, NDA, sNDA, IMPD, and ANDA filings for the FDA and European regulatory agencies. Dr Brinkman has commercially launched nine products encompassing oncology, metabolic, dermatology, and endocrinology therapeutic areas and contributed to ANDAs for various semi-solid and parenteral products. He is also an inventor on 14 patents. His expertise includes current Good Manufacturing Practice (cGMP) systems applied to API manufacture / Drug Product manufacture and addressing regulatory issues. Previously, Dr Brinkman was Executive Director of Product Development at Arcutis Biotherapeutics, Inc. where he was responsible for the successful commercial launch of ZORYVE (roflumilast). Dr Brinkman earned his undergraduate degree from Rutgers University and a PhD in Organic Chemistry from Seton Hall University.

Holli Cherevka
Clinical Operations
Read more
Ms Cherevka has more than 15 years of leadership experience in clinical development, clinical operations, project/program management, CMC and quality. She serves on the Board of Directors for Artyc, a company focused on decarbonizing cold chain logistics in pharmaceutical development. Prior roles include Chief Operating Officer for Ampio, a publicly traded development-stage pharmaceutical company and Director of Business Development for the American College of Radiology imaging core lab for drug, biologic, and medical device development. Ms Chervka has a MSc Biomedical and Molecular Sciences Research, King’s College, London.

Genmin Lu, PhD
Translational Medicine
Read more
Dr Lu has over two decades of R&D experience, with expertise in translational medicine and drug discovery. He invented ANDEXXA, a biologic for hemorrhage associated with FXa inhibitors, leading the program from discovery through regulatory approval while at Portola Pharmaceuticals. He was also the Director of Translational Medicine at Alexion, AstraZeneca Rare Disease. Dr Lu holds over 40 patents and has published numerous papers in journals including Nature Medicine and the New England Journal of Medicine. Dr Lu received his PhD in Physical Chemistry and had his postdoc training in enzymology and protein biochemistry.

Nathan Ogden, PhD
CMC and Quality
Read more
Dr Ogden has experience in regulatory, CMC and quality control for small molecule and polymer-based hematological drugs. Dr Ogden earned his undergraduate degree from Rice University and a Ph.D. in Materials Science from the University of California, Santa Barbara.

Kyle Ploense, PhD
Preclinical Research
Read more
Dr Ploense co-founded Cayuga Biotech with Dr Kudela. He has held several research positions at the University of California, Santa Barbara, University of Colorado, Boulder and is currently Associate Scientist at the Icahn School of Medicine at Mount Sinai. He earned an undergraduate degree and a Ph.D. in Psychological and Brain Sciences from the University of California, Santa Barbara.

Charlie Pollack, MD
Clinical Development
Read more
Dr Pollack has more than 30 years of clinical/research experience in emergency medicine. He has served a PI or co-PI on multiple trials in the thrombosis and antithrombotic reversal spaces, is the author of more than 500 original research papers, serves on the editorial boards of several journals and is on the steering committees of multiple national and international trials. Previously Dr Pollack was on active duty in the US Navy Medical Corps and was trained in combat casualty care. He earned his undergraduate degree from Emory University and his medical degree from Tulane University.

Ajay Rai
Corporate Development
Read more
With over 25 years of biopharmaceutical industry experience, Mr Rai has executed over 30 in-licensing, out-licensing, R&D collaborations, acquisitions, and divestments totaling over $7B+ in transaction value across multiple therapeutic areas and technologies, and raised over $375M+ in equity and non-dilutive financing from venture capital, strategic and public investors for emerging biotechs. Ajay is currently an advisor and consultant to multiple platform and clinical stage biotechnology companies. He previously held business development and strategy positions at Sigilon Therapeutics (acquired by Eli Lilly), Frequency Therapeutics, Biogen, Forest Labs and Takeda. He earned a B.S. in Finance from New York University’s Stern School of Business and a M.B.A. from Case Western University.

Monil Shah, PhD
Clinical Development
Read more
Dr Shah has over 25 years of pharmaceutical industry experience in drug development with multiple research and clinical development roles at companies including Novartis, Amgen, Celgene, BMS, Brooklyn ImmunoTherapeutics and WindMIL Therapeutics. He is currently Managing Director at IAA Consulting.

Kip Vought
Regulatory
Read more
Mr Vought has over 30 years pharmaceutical experience with all stages of development including clinical, nonclinical, CMC, and regulatory expertise. He held positions at IVAX, NaPro BioTherapeutics, Regulus/Clinpace, and Scilex Pharmaceuticals, and is currently a Partner at IAA Consulting.

Shiyin Yee, PhD
Clinical Pharmacology
Read more
Shiyin Yee earned a PhD in Pharmaceutics from the University of Michigan. She has over 30 years of experience in drug development, including multiple successful INDs, ANDAs, NDAs and MAAs. Her experience managing both preclinical and clinical pharmacology programs spans multiple therapeutic areas, including thrombosis and hemostasis.
*Cayuga employees; all others are consultants/advisors
Advisors

Joshua Goldstein, MD, PhD
Read more
As Professor of Emergency Medicine at Harvard Medical School, and MGH Endowed Chair in Emergency Research in the Department of Emergency Medicine at Massachusetts General Hospital, Dr Goldstein’s areas of focus include blood coagulation, anticoagulation reversal, intracerebral hemorrhage and stroke. He has acted as PI for multiple studies within the hemostasis and thrombosis space and has more than 250 research articles in the literature to his credit. Dr Goldstein received his undergraduate degree at Tufts and his MD and PhD in Molecular Biology at University of Connecticut. His postdoctoral work was at the Center for Hemostasis and Thrombosis, Beth Israel Deaconess Medical Center.

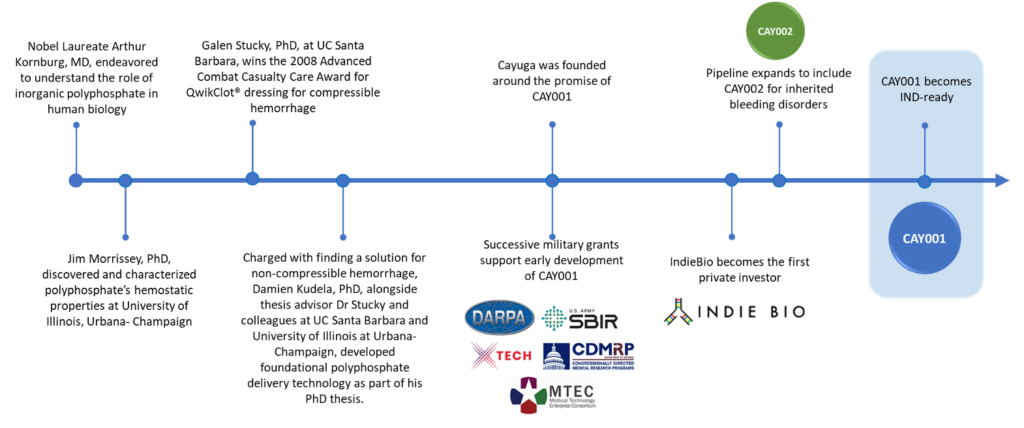
James Morrissey, PhD
Read more
Dr. Morrissey is a Professor of Biological Chemistry and Internal Medicine at the University of Michigan Medical School and recently completed his term as Editor-in-Chief of the Journal of Thrombosis and Haemostasis. His research has focused on biochemical mechanisms that regulate the blood clotting system. His laboratory discovered that inorganic polyphosphate, which is released from activated human platelets, is a potent modulator of blood clotting and inflammation and the healing response.

Galen Stucky, PhD
Read more
Dr Stucky is Distinguished Professor and Essam Khashoggi Chair in Material Chemistry at the University of California Santa Barbara. His areas of research focus include nanotechnology and biosystem processes such as the blood clotting cascade and hemostasis. Dr Stucky has been ranked in the top five most-cited materials scientists in the world. He has won numerous awards for his work, including the Advanced Technology Applications for Combat Casualty Care Award for the development of KuikClot Hemostatic Dressing. Dr Stucky was elected a member of the American Association for the Advancement of Science, a member of the American Academy of Arts and Sciences, and a member of the National Academy of Sciences. After receiving his undergraduate degree from McPherson College, he received his
PhD in Physical Chemistry from Iowa State University and completed postdoctoral study at MIT and the Quantum Chemistry Institute.

Karen Boezi
Read more
Karen Boezi has been involved in building, leading and investing in biopharmaceutical companies for more than 30 years. Karen is currently the COO of Thirona Bio, a clinical stage company with a skin fibrosis drug completing a Phase 1B human clinical study. Previously, Karen was the CEO of Redwood Bioscience, founded on technology from Nobel Laureate Carolyn Bettozzi’s lab, which she led through its successful sale to Catalent (CTLT) under a staged acquisition option. Redwood, now a division of Novo Holdings, continues to be a pioneer in developing antibody-drug conjugates (ADCs) and toxin-linker-conjugation technology for improving oncology care, with two clinical stage products incorporating its SMARTag technology.
Prior to joining Redwood, Karen was a founding Partner of Thomas, McNerney & Partners (TMP), a health care technology-focused venture firm with $600 million under management. She launched TMP with a colleague from Warburg, Pincus, where she was part of the seed stage life science team, and a partner from Coral Ventures, where she was a General Partner. TMP was recognized for managing one of the top performing 2006 vintage venture funds globally. During her venture career, she invested in 15 companies that went public and/or were acquired. Karen began her career as a member of Alex. Brown & Sons’s health care and insurance corporate finance teams. More recently, Karen has been an angel investor and advisor to academic scientists founding biopharmaceutical companies, including Design Therapeutics (DSGN) and Acrigen Bioscience, a gene-editing company on whose Board she serves. She graduated Phi Beta Kappa with a B.S. in Economics from the Wharton School at the University of Pennsylvania.
Board of Directors

Andrea Ashford-Hicks
Chief Executive Officer
Read more
Ms Ashford-Hicks has more than 20 years of biopharmaceutical industry experience spanning multiple product launches, IND, BLA and NDA approvals as well as business development transactions. Prior to joining Cayuga, she led the BLA approval and commercial launch of RETHYMIC® at Enzyvant Therapeutics. Previously, Ms Ashford-Hicks held roles of increasing responsibility at Johnson & Johnson, Pfizer, Agenus, EMD Serono and Schering-Plough (now Merck). She has degrees in Biology and Finance from the University of Pennsylvania’s College of Arts and Sciences and The Wharton School of Business.

Damien Kudela, PhD
Chief Science Officer
Read more
Dr Kudela is the founder of Cayuga and inventor of CAY001. He received his PhD in Materials Chemistry from the University of California, Santa Barbara. He graduated from Cornell University with a BA in Chemistry, cum laude, and a BA in History. He graduated from Deerfield Academy. Prior to founding Cayuga Biotech, Dr. Kudela interned with the US Department of State.

John Curnutte, MD, PhD
Read more
Dr Curnutte brings over 30 years of biopharmaceutical leadership experience to Cayuga. A pediatric hematologist-oncologist and biochemist, he previously led Research and Development from 2011 until his retirement in 2019 at Portola Pharmaceuticals (acquired by Alexion and then AstraZeneca) where he was responsible for bringing two medicines in the hemostasis and thrombosis space, andexanet alfa and betrixaban, through FDA approval and commercial launch. Prior to joining Portola, Dr Curnutte served as Chief Executive Officer of 3-V Biosciences, a biotechnology company focused on host-directed antiviral medicines. From 2000 to 2008, he was President of Schering-Plough Biopharma (formerly DNAX Research Institute and now Merck Research Laboratories) where his teams discovered IL-17 and IL-23. Dr Curnutte was Head of Immunology at Genentech from 1993 to 2000 where his department played important roles in the development of Rituxan, Herceptin, and Xolair. During this period, he was also an adjunct clinical professor of pediatrics at Stanford University School of Medicine and a member of the medical staff from 1993 to 2013. Prior to Genentech, Dr Curnutte was a tenured faculty member at The Scripps Research Institute pursuing basic and clinical research in inflammation biochemistry and the molecular genetics of congenital immune deficiencies. Dr Curnutte is a member of the Board of Directors at Pliant Therapeutics and previously, Orchard Therapeutics (acquired by Kyowa Kirin in 2024) and Diadexus. He is also an advisor to Samsara BioCapital and a Scientific Advisory Board member at Autobahn Labs. Dr Curnutte holds an A.B. in Biochemistry and Molecular Biology from Harvard University and an M.D. and a Ph.D. in Biological Chemistry from Harvard Medical School.

Silvia Codony, MSc. MBA
Board Observer
Read more
Ms Codony is a venture investor, entrepreneur, and life sciences executive with over 20 years of experience in medical devices, biotech and digital health. She currently serves as Assistant Vice President, NY Ventures at Empire State Development. Before becoming a venture investor, she co-founded a company in digital health and held several leadership and consultant roles in the industry, including biotech, medical device and digital health companies across therapeutic areas such as cardiology, urology, plastic surgery, and diabetes among others. Silvia started her career in Europe and moved to the States years to build the US affiliate for a European medical device company. She has worked in sales and business development, has a technical background in clinical and regulatory affairs and holds an MSc in Mathematics and an executive MBA from IE Business School.

Steve Gormicans, MD
Board Observer
Read more
Dr Gormican brings more than 35 years healthcare experience to Cayuga’s Board. Dr Gormican is Chief Medical Officer at Eyegenex, Inc and Member of the NuFund Venture Group. Previously, he served as Chairman of the Department of Emergency Medicine, Chief Financial Officer and Chairman of an Institutional Review Board at Scripps Memorial Hospital – La Jolla. He developed and published the widely cited CRAMS scale for field triage for trauma victims. Previous roles include Board Director for Eumentis Therapeutics and Casana and Chief Medical Officer for RockWest and Consuli. Dr Gormican earned his undergraduate degree at Boston College and his Medical Degree at the University of Toronto.
Investors






